View pictures in App save up to 80% data.
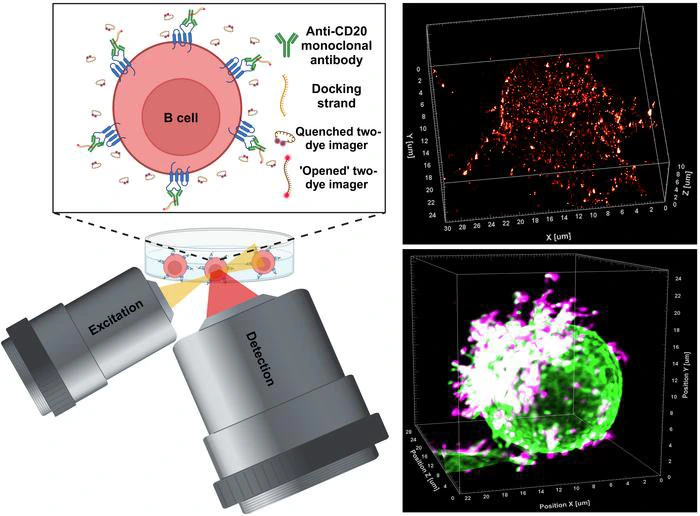
In the field of medical research, an innovative advancement has emerged from the University of Würzburg's laboratories in Bavaria, Germany. The study led by Professor Markus Sauer and his team represents a significant progression in the comprehension and creation of therapeutic antibodies. This research sheds light on the complex interactions between antibodies and their target molecules, especially in relation to B cells linked to blood cancers, including chronic lymphocytic leukaemia. The research team has pioneered a cutting-edge super-resolution microscopy method called LLS-TDI-DNA-PAINT, which enables real-time visualization of therapeutic antibodies attaching to target proteins on cancer cells in three-dimensional space, achieving an unprecedented level of detail and precision.
For many years, immunotherapeutic antibodies have played a crucial role in the treatment of different types of tumors. A key focus of these therapies is the CD20 protein found on the surface of B cells. The binding of these antibodies to CD20 triggers a series of immune responses that ultimately result in the elimination of cancerous cells. Nonetheless, even with the proven effectiveness of these antibodies in clinical settings, scientists have struggled to fully understand how they bind and the cellular responses that follow. Recent groundbreaking research from Würzburg has made significant strides in addressing this knowledge gap.
Using the LLS-TDI-DNA-PAINT method, scientists successfully observed the real-time dynamics of therapeutic antibodies. They examined the interaction between these antibodies and CD20 molecules, focusing on how this binding affects the structural behavior of B cells during the process. A significant finding from the study was the observation of a phenotypic change in B cells, which the researchers referred to as adopting a “hedgehog shape.” This alteration occurs due to the crosslinking of CD20 proteins by the antibodies, which activates complement systems and enhances targeted immune responses.
The researchers performed their observations utilizing the Raji B cell line, which is derived from human Burkitt's lymphoma and is well-regarded for its significance in cancer research. This specific cell line acts as a model for evaluating B cell responses to therapeutic antibodies. The research team executed experiments with four different therapeutic antibodies: RTX, OFA, OBZ, and 2H7, all of which are recognized for their targeting of CD20. The findings from their studies indicated that, irrespective of the antibody type used—whether categorized as type I or II—the response mechanism was remarkably similar, challenging earlier beliefs held by the scientific community.
Additionally, research has demonstrated that therapeutic antibodies primarily bind to specific areas of the B cell membrane, particularly at microvilli. These tiny projections serve as crucial sites for protein interactions and contribute to the overall stability of the B cell surface during therapeutic applications. The noted polarization of B cells, alongside the reinforcement of microvilli, supports the idea that the structure of the cell membrane significantly influences immune responses. These insights not only clarify the processes involved in antibody binding but also suggest opportunities for enhancing therapeutic approaches by adjusting these interactions.
An additional noteworthy feature of this research is the possible role of hedgehog-shaped B cells in enhancing inter-cellular communication within the immune system. The scientists suggest that this change in shape could be crucial for the development of immunological synapses, encouraging B cells to interact with macrophages and natural killer cells. This hypothesis expands on existing knowledge and highlights the urgent need for more investigations into how these activated B cells contribute to the coordination of larger immune responses.
The introduction of the LLS-TDI-DNA-PAINT method marks a significant advancement in our ability to investigate therapeutic mechanisms at the molecular scale. This innovative technique lays the groundwork for future research focused on understanding the intricacies of cell-surface interactions and the complex processes that underpin immune recognition and the elimination of cancer cells. With this state-of-the-art visualization technology, scientists are empowered to examine the subtle nuances of antibody binding dynamics, creating an exceptional foundation for the development of tailored immunotherapy strategies.
As the research advances, the team at the University of Würzburg aims to explore further the potential impacts of their discoveries on the future of antibody-driven cancer treatments. Thanks to the breakthroughs achieved with the LLS-TDI-DNA-PAINT method, there is an encouraging outlook where therapeutic antibodies can be customized and developed by directly analyzing their behavior within the tumor microenvironment.
In conclusion, this pivotal study from Würzburg not only disputes current models in the therapeutic antibody sector but also highlights the transformative potential of advanced microscopy methods in reshaping our comprehension of cancer biology. As scientists persist in their investigations, the optimism for better therapeutic approaches and enhanced patient results is strong, serving as a source of inspiration in the relentless fight against blood cancers and other related diseases.
Subject of Research: Cells
Article Title: Decoding the molecular interplay of CD20 and therapeutic antibodies with fast volumetric nanoscopy
News Publication Date: 9-Jan-2025
Web References: Journal Article
References: N/A
Image Credits: Arindam Ghosh / University of Wuerzburg
Keywords: super-resolution microscopy, therapeutic antibodies, B cells, CD20, cancer research, immunotherapy, LLS-TDI-DNA-PAINT, chronic lymphocytic leukaemia, Burkitt’s lymphoma, immune response, molecular interactions.
Explore further insights from the world of Science.
Sign up to receive the newest posts directly in your inbox.